
ICP-MS (Thermo-Fisher Element 2)
Description
The Thermo-Fisher Element 2 double focusing sector field ICP-MS performs elemental analyses of trace elements in aqueous solutions and, when used in combination with a laser, major and trace element analyses in solids and glasses, as a method known as laser-ablation ICP-MS.
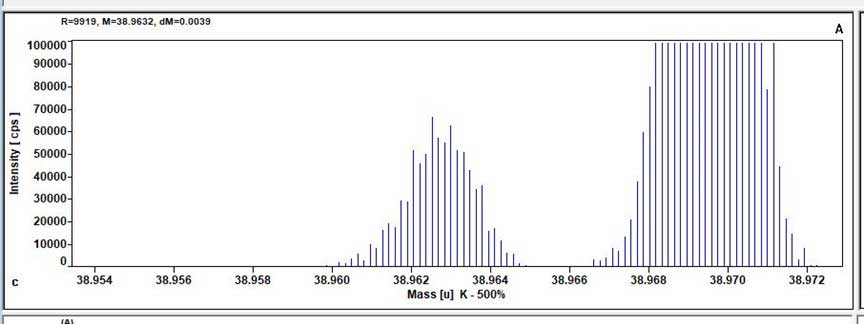
It has a high mass resolution capability, allowing to resolve isobaric interferences between isotopes of interest and polyatomic compounds such as oxides, hydrides, etc…This capability is particularly important for precise and analysis of natural waters main cations, and many toxic metals such as As and Se. When the elements of interest do not require such high mass resolution, the Element 2 can analyze elements with a higher sensitivity and better precision than conventional (quadrupole) ICP-MS.
Below are the general sample requirements for aqueous solutions for ICP-MS. For LA-ICP-MS, please consult our specific laser page.
Sample Preparation
Samples must be digested and free of particulate matter, organic or inorganic. Proper digestion methods will vary depending on materials. Please consult with us for guidance.
Most elements are more stable as dissolved cations in nitric acid solutions, and calibration standards are commercialized this way. It is therefore preferred that users acidify their samples to a ~1-2% NHO3 solution, using acid and water of high purity grades (trace metal grade and nanopure).
It is strongly recommended that users provide with their samples a procedural blank, which has been digested and diluted the exact same way as the samples, using the same solvents, so to rule out potential contamination processes.
A minimum sample volume of 2 mL is required, but a volume of ~5-15 mL is preferred, as this gives the option to re-analyze samples if needed, and allows to randomly repeat analyses for various purposes incuding testing for sample homogeneity. 15 mL centrifuge tubes are preferred, although other sample vial types can be accommodated.
Samples are run after spiking with an internal standard of known concentration, either in the ICP-MS laboratory, or by the user in their laboratory after following our instructions. We provide internal standard and calibration standards, but users are encouraged to also provide their own calibration standards and/or standards to be run as unknowns (secondary standards), if they desire.

Examples of Element 2 ICP-MS Analysis Signal
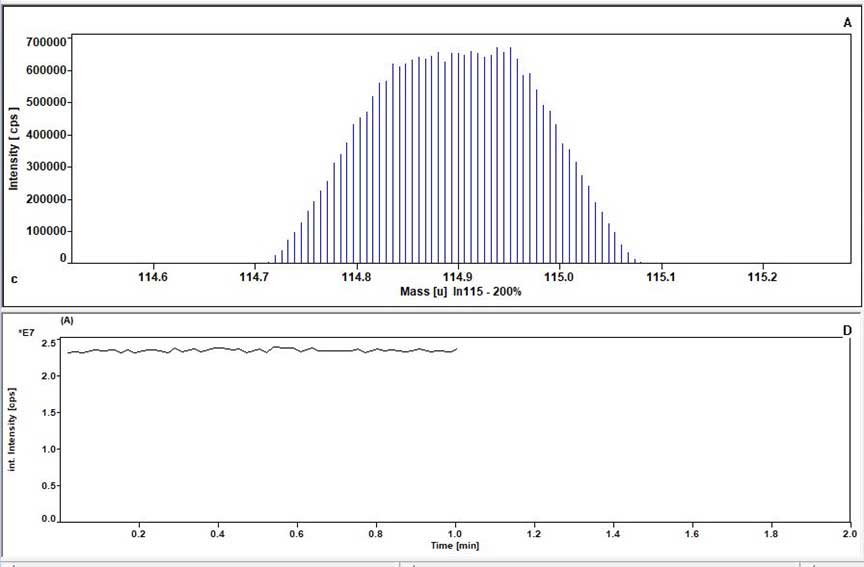
Upper: Example of a signal obtained in normal (low) resolution for indium in concentration of 1 ppb. The Element 2 allows for detailed mass scans of each isotope, allowing for tuning of optimal ion beam conditions and the generation of a flat-topped peak over a wide mass range, as shown here.
Lower: integral of the signal obtained from the above peak over 1 minute of acquisition, highlighting the instrument stability over a typical sample analysis time)
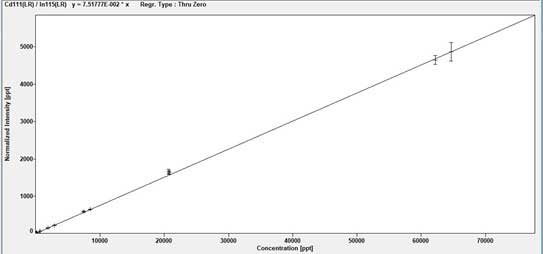
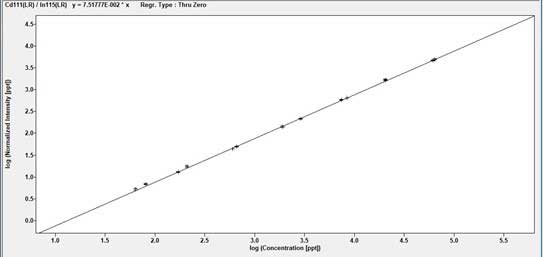
Examples of Trace Element Analysis Calibration
Contact and Pricing
Charge per sample is commensurate with number of elements requested, from a minimum charge of $25 for one element (NIU rate). We offer “packages” such as major cations ($30/sample -NIU rates-) or EPA-regulated toxic trace metals ($40/sample -NIU rates-), or any user-customized element list, which may include up to ~60 elements. Please inquire with your element list for a quote.
| Description | NIU | Academic | Commercial |
|---|---|---|---|
|
Instrument use |
$25-100 |
$30-120 |
$50-200 |
|
Technician time * |
N/A |
N/A |
$100 |
|
Minimum fee for new project ** |
$300 |
||
* Technician time is charged for projects which need extra sample processing time due to improper preparation, or for samples of unknown composition that may require a preliminary qualitative analysis.
** To reflect the low sample throughput and method development inherent to projects that are new to us, a flat rate of $300 is charged in lieu of the fees per sample, should these amount <$300. Returning customers repeating an existing method on additional samples will be charged per sample.
Contact Guillaume Girard at ggirard@niu.edu for inquiries and questions.
Contact Us
Micro-Compositional Analysis LaboratoryJoshua Schwartz
Laboratory Manager
joshua.schwartz@niu.edu
815-753-7930