Douglas A. Klumpp
Representative Publications
“Electrophilic Carboxamidation of Ferrocenes with Isocyanates.” Qurah, A.; Schaeff, M. N.; Kethe, A.; Klumpp, D. A. J. Org. Chem. 2017, 82, 10623–10627. DOI 10.1021/acs.joc.7b01347.
“Conjugate Addition of Amino Acids and Alkenyl‐Substituted N‐Heterocycles.” Kennedy, S. H.; Klumpp, D. A. J. Org. Chem. 2017, 82, 10219–10225. DOI: 10.1021/acs.joc.7b01724.
“Synthesis of Heterocycle‐Containing 9,9‐Diarylfluorenes Using Superelectrophiles.” Gasonoo, M.; Sumita, A.; Giuffre, K.; Boblak, K.; Ohwada, T.; Klumpp, D. A., J. Org. Chem. 2017, 82, 6044–6053. DOI: 10.1021/acs.joc.7b00311
“Use of charge–charge repulsion to enhance π‐electron delocalization into anti‐aromatic and aromatic systems” Sumita, A.; Ohwada, T.; Boblak, K.; Gasonoo, M.; Klumpp, D. A., Chem. Eur. J. 2017, 23, 2566–2570. DOI: 10.1002/chem.201606036.
“Cyclizations of N‐Carbamoyl and N‐Thiocarbamoyl Iminium Ions Leading to Ring‐Fused Heterocycles.” Qurah, A.; Klumpp, D. A., Tetrahedron Lett., 2016, 57, 4788–4790.
“Intramolecular Reactions of Furan‐based bis‐(p‐Quinodimethanes)” Klumpp, D. A.; Gilbert, T. A.; Trahanovsky, W. S. Eur. J. Org. Chem. 2016, 2016(33), 5559–5568. DOI: 10.1002/ejoc.201600995
“Tetra‐ and Pentacationic Electrophiles and Their Chemistry” Gasonoo, M.; Naredla, R., R.; Nilsson Lill, S. O.; Klumpp, D. A., J. Org. Chem. 2016, 81, 11758–11765 DOI 10.1021/acs.joc.6b02220.
“Superacid‐promoted synthesis of polychlorinated dibenzofurans.” Qarah, A.; Gasonoo, M.; Do, D.; Klumpp, D. A. Tetrahedron Lett., 2016, 57, 3711–3714.
“Intramolecular Reactions of Thiophene‐based bis‐(p‐Quinodimethanes).” Trahanovsky, W. S.; Klumpp, D. A., Tetrahedron Lett., 2016, 57, 2386–2389.
Synthetic and Mechanistic Organic Chemistry
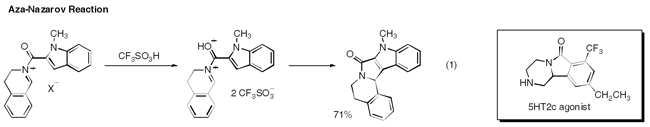
Our research group works within the field of organic chemistry. We have interests in the development of new synthetic methods, often utilizing strong electrophiles and acid systems. The development of synthetic methodologies is often coupled with applications in target‐directed organic synthesis. For example, we recently described the superacid‐catalyzed aza‐Nazarov reaction, eq 1 (Organic Letters 2007, 9, 3085). This chemistry is presently being applied to the synthesis of Bristol–Myers Squibb's 5HT2c agonist.
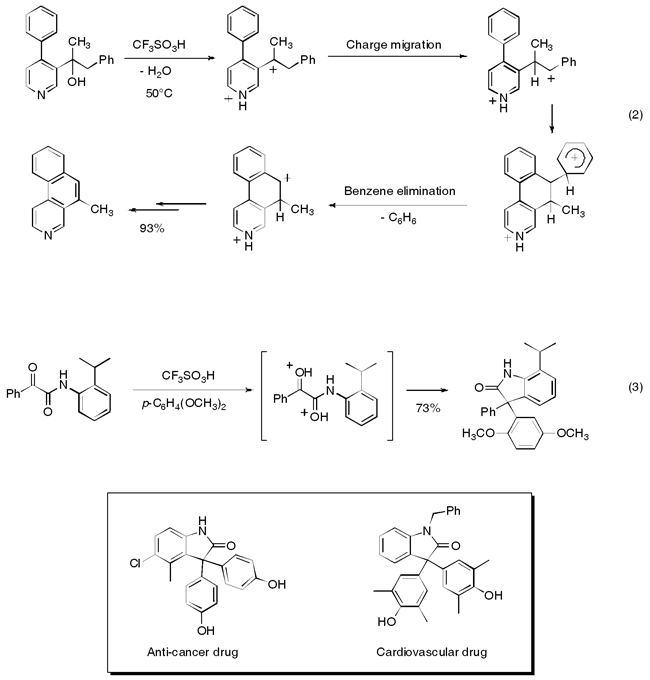
Our expertise in acid‐catalyzed reactions has enabled us to develop several new methods for the synthesis of novel heterocyclic products. With unusual charge migration and benzene elimination steps, we were able to prepare a series of functionalized aza‐polycyclic aromatic compounds, such as the benzo[f]isoquinoline, eq 2 (Organic Letters 2006, 8, 1233 and J. Org. Chem. 2008, 73, 3654). We also prepared 2‑oxyindoles by the reactions of α‑ketoamides in superacid, eq 3 (J. Org. Chem. 2008, 73, 6506). Since the 2‑oxyindole ring system is present in a large number of biologically active compounds, this chemistry represents a useful new synthetic methodology.
Our mechanistic studies involve the study of structure–reactivity relationships in electrophiles and the characterization of highly reactive electrophiles. For example, we recently reported that dicationic electrophiles, or superelelctrophiles, may undergo single electron transfer (SET) reactions with a suitable electron donor, eq 4 (J. Am. Chem. Soc. 2008, 130, 14388). The one electron reduction leads to radical cation chemistry, and in the case of the oxazole derivative, a dimerization pathway.
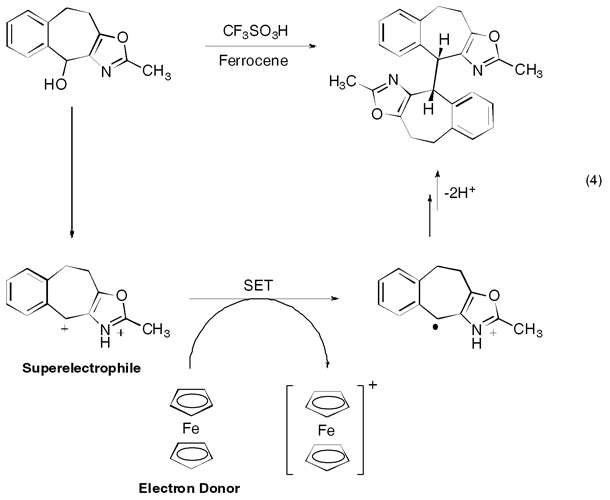
Figure 1. A pictorial image of single electron transfer to the superelectrophile.
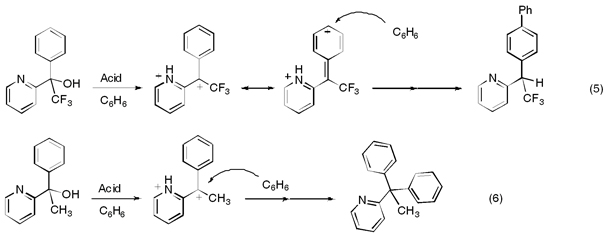
We have also examined the factors that tend to favor the migration of charge across organic structures. This chemistry is fundamentally important in the design of molecular electronics, but it can influence the regiochemistry of some reactions. Recent work has shown that the trifluoromethyl group (‑CF3) can exert an effect that drives the cationic charge center across the phenyl ring, eqs 5 and 6 (J. Am. Chem. Soc. 2010, 132, 3266). Consequently, nulceophilic attack occurs at the distant site due to charge migration (eq 5), while a similar reaction involving a methyl group shows no tendency to undergo charge migration (eq 6).
New research projects in our group include the development of asymmetric catalytic methods, the development of green chemistry and polymer synthesis. Groups members will also continue studies of structure–activity.

Presidential Research, Scholarship and Artistry Professor
Faraday Hall 356
815‐753‐1959
dklumpp@niu.edu
www.niu.edu/klumpp
Educational Background
Visiting Scholar, University of Southern California, Loker Hydrocarbon Research Institute and Department of Chemistry, 1996–2003
Postdoctoral Fellow, University of Southern California, 1993–1996
Ph.D., Iowa State University, 1993
B.S., University of Oklahoma, 1987
Research Interests
Organic Synthetic Methods Development: utilization of novel electrophiles; heterocyclic chemistry and synthesis; pharmaceuticals; chemistry at non‐activated carbon; asymmetric catalysis; polymers; synthesis with bio‐renewable feedstock.
Organic Mechanistic Studies:reactive intermediates; use of kinetic studies, theoretical calculations, and spectroscopic methods.