Oliver Hofstetter
Representative Publications
Hofstetter, O.; Mlakar, A.; Roux, C.; Spindler, X.; Lennard, C. Fingermark Lifting and Visualization Device and Methods of Use. U.S. Patent 10,866,188 issued December 15, 2020.
Lam, R.; Hofstetter, O.; Lennard, C.; Roux, C.; Spindler, X. Evaluation of Multi-Target Immunogenic Reagents for the Detection of Latent and Body Fluid-Contaminated Fingermarks. Forensic Sci. Int. 2016, 264, 168-175.
Davenport, K. R.; Smith, C. A.; Hofstetter, H.; Horn, J. R.; Hofstetter, O. Site-Directed Immobilization of a Genetically Engineered Anti-Methotrexate Antibody Via an Enzymatically Introduced Biotin Label Significantly Increases the Binding Capacity of Immunoaffinity Columns. J. Chromatogr. B 2016, 1021, 114-121.
Eleniste, P.; Hofstetter, H.; Hofstetter, O. Expression and Characterization of an Enantioselective Antigen-Binding Fragment Directed Against α-Amino Acids. Prot. Expr. Pur. 2013, 91, 20-29.
Kassa, T.; Undesser, L. P.; Hofstetter, H.; Hofstetter, O. Antibody-Based Multiplex Analysis of Structurally Closely Related Chiral Molecules. Analyst 2011, 136, 1113-1115.
Spindler, X.; Hofstetter, O.; McDonagh, A.; Roux, C.; C. Lennard, C. Enhancement of Latent Fingermarks on Non-Porous Surfaces Using Anti-L-Amino Acid Antibodies Conjugated to Gold Nanoparticles. Chem. Commun. 2011, 47, 6602-5604.
Franco, E. J.; Hofstetter, H.; Hofstetter, O. Determination of Lactic Acid Enantiomers in Human Urine by High-Performance Immunoaffinity LC-MS. J. Pharm. Biomed. Anal. 2009, 49, 1088-1091.
Hofstetter, H.; Hofstetter, O. Antibodies as Tailor‐Made Chiral Selectors for Detection and Separation of Stereoisomers. Trends Anal. Chem. 2005, 24, 869–879.
Development of (Bio)analytical Techniques
Physiological processes invariably depend on specific interactions between biological binding partners. These interactions can be exploited in a great variety of analytical techniques for the targeted purification or detection of biological macromolecules and small ligands. The Hofstetter group has a long history of developing chromatographic systems, assays, and sensors, which includes the generation of highly selective antibodies (using both classical and molecular biological approaches), the investigation of various solid phases (e.g., gold, natural and synthetic polymers), and the search for appropriate immobilization and derivatization methods (both chemical and enzymatic). Figures 1 and 2 show examples of the application of solid-phase immobilized antibodies for the separation of drugs and the enantiomers of metabolites, respectively, by immunoaffinity chromatography.
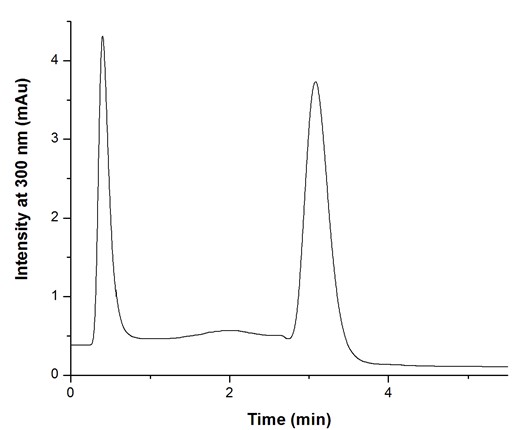
Fig. 1: Chromatographic separation of the antifolates aminopterin (first peak) and methotrexate (second peak) at 1 ml/min on a column produced by immobilizing a genetically engineered camelid anti-methotrexate antibody on a high-flow-through support material. The baseline disturbance indicates the time when the mobile phase changed from phosphate-buffered saline, pH 7.4, to acetate buffer pH 4, for elution of methotrexate.
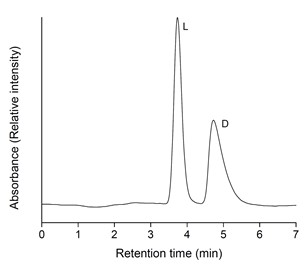
Fig. 2: Enantiomer separation of D, L-lactic acid by high-performance immunoaffinity chromatography using a monoclonal anti-D-hydroxy acid antibody as chiral selector. The flow rate was 0.5 ml/min, the solvent phosphate-buffered saline, pH 7.4.
More recently, antibody-based and chemical methods have also been utilized for applications in forensic science. For example, the Hofstetter group has developed a new technique for lifting and visualizing latent fingermarks, which is based on the reaction of pH-indicators – immobilized on suitable solid supports such as nylon membranes – with acids in fingermark deposits (e.g., lactic acid). The exposure of appropriate pH-sensitive reagents to such an environment causes a change in their spectroscopic properties, which can be seen, depending on the type of indicator, either under ambient or luminescent light conditions. These easy-to-use lifters can detect marks from a wide range of ages and a great variety of surfaces. (Figure 3).

Fig. 3: Fingermarks lifted from a) plastic, b) glass, c) a glossy magazine, d) printer paper, and e) wood using a pH-sensitive lifter. Visualization was achieved under ambient light (a) and at 445 nm excitation coupled with a 550 nm bandpass filter, respectively (b-e).
Research in Dr. Hofstetter’s laboratory is interdisciplinary and combines aspects of immunology, protein chemistry, analytical chemistry, molecular biology, and forensic science.

Associate Professor
La Tourette Hall 428
815‐753‐6898
ohofst@niu.edu
Educational Background
Postdoctoral Fellow, Weizmann Institute of Science, 1999–2000
Dr. rer. nat., Eberhard‐Karls‐Universität Tübingen, 1999
M.S., Eberhard‐Karls‐Universität Tübingen, 1995
B.S., Eberhard‐Karls‐Universität Tübingen, 1991
Research Interests
Research in Dr. Hofstetter’s laboratory is interdisciplinary and combines aspects of immunology, protein chemistry, analytical chemistry, molecular biology, and forensic science.