- Zhili Xiao, Ph.D.
- Projects
- Hydrogen Gas Sensors
Hydrogen Gas Sensors
Effective and reliable sensors are essential in the implementation all aspects of a hydrogen economy. As a major part of the hydrogen economy, fuel cell economy requires hydrogen sensors with extremely fast response for process monitoring and extremely high sensitivity for leak detecting. Due to the mobile and compact nature of automotive fuel cells, hydrogen sensors have to be small and integrative. Currently, commercial sensors suffer from slower response times than the likely duty cycles needed for most applications. The large size of the available sensors is also not suitable for automotive fuel cell applications. Recent research in mesoscopic hydrogen sensors, however, have shown promising results in hydrogen sensing, with response times down to tens of milliseconds.
We aim to utilize our strengths in nanofabrication to develop hydrogen sensors with high selectivity, high sensitivity, high integrity, fast response and minimum size. Since 2004 we have worked on new generation of hydrogen sensors based on palladium nanostructures:
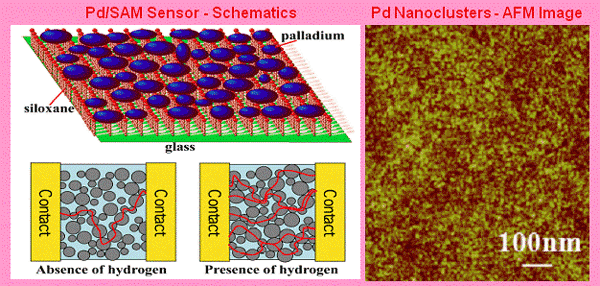
- Ultra-thin (less than 5 nm) palladium (Pd) film on glass substrates coated with siloxane self assembled monolayers (SAMs). SAMs promote the formation of Pd nanoclusters and reduce the friction between Pd and the substrate. This type of hydrogen sensors can have a response time as short as 75 milliseconds in detecting 2% hydrogen gas [T. Xu et al., Applied Physics Letters 86, 203104 (2005)].
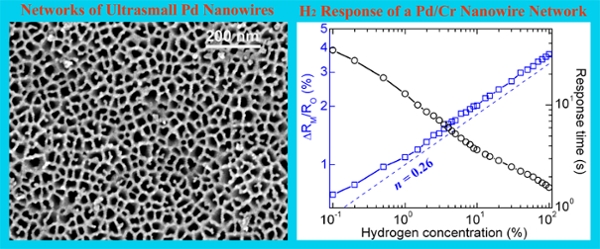
- Networks of ultra-small nanowires of Pd, Pd-alloy, Pd/metal bilayer and multilayers formed on commercially available filtration membranes. These hydrogen sensors take advantage of single palladium nanowires in high speed and sensitivity and that can be fabricated conveniently. Furthermore, the confinement-induced suppression of a phase transition in the Pd/H system enables the performance of H2 sensors go beyond the benefits expected from the increased surface area to volume (SA/V) ratios and shorter diffusion distances. That is, this new type of hydrogen sensors can distinguish H2 concentrations up to 100%, eliminating a crucial drawback of its pure palladium counterparts. [X. Q. Zeng et al., Nano Letters 11, 262 (2011); ACS Nano 5, 7443 (2011)].