Chemistry of Superelectrophiles
The Klumpp research group is well known for their synthetic and mechanistic work with superelectrophiles. Superelectrophilic chemistry was first developed in the mid-1970s by Professor George A. Olah (1994 Chemistry Nobel Prize) and coworkers. Following postdoctoral work in the Olah group, Professor Klumpp began his own studies in this area in the late 1990s. Professors Olah and Klumpp coauthored a book on this topic in 2008, Superelectrophiles and Their Chemistry (Wiley & Sons, NY).

Recent work with superelectrophiles has involved the development of several new synthetic methodologies. Superelectrophiles are inherently reactive chemical species and this reactivity may be exploited in synthetic conversions.
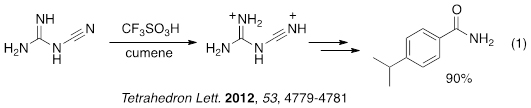
For example, direct carboxamidations of arenes using Friedel-Crafts chemistry is not readily accomplished. We showed that this conversion may be done using the inexpensive reagent, cyanoguanidine (eq 1). The conversion is thought to proceed through the deprotonated, superelectrophilic species.
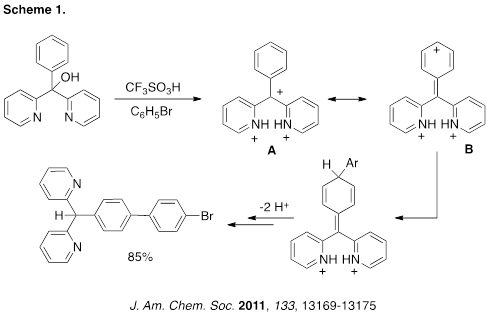
Charge-charge repulsive effects are known to be important in superelectrophiles. We have used this effect to accomplish remote functionalization of a carbocation (Scheme 1). Thus, ionization of the triarylmethanol leads to the tricationic species, where charge-charge repulsion leads to major contributions from resonance structure B. Nucleophilic attack at the remote position leads to the final product in 85% yield.
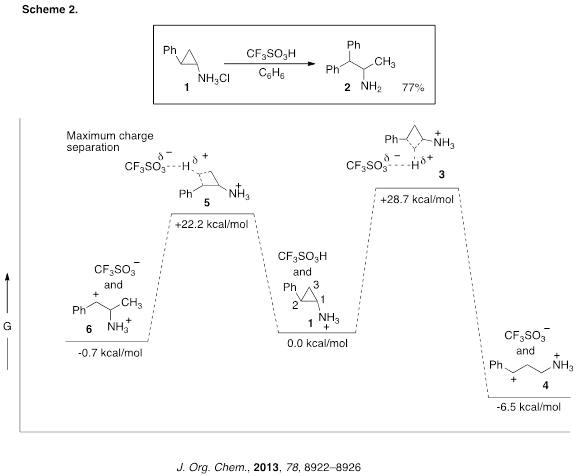
We have recently described an example of a superelectrophilic alkylation in which charge-charge repulsive effects may be important in the kinetic control of a reaction. Thus, reaction of compound 1 (tranylcypromine, a clinically useful anti-depressant drug) leads to ring opening of the C2-C3 bond and formation of product 2 in 77% yield (Scheme 2). Calculations showed that protolysis of the C1-C2 bond (3) would provide a fairly stable 1,4-dication (4), while protolysis of the C2-C3 bond (5) would gives the less stable 1,3-dication (6). Dication 6 leads to product 2 by subsequent Friedel-Crafts chemistry. In order to explain this observation, we reasoned that protolysis of the C2-C3 bond is favored due to separation of the cationic charges – leading to kinetic control of the transformation.
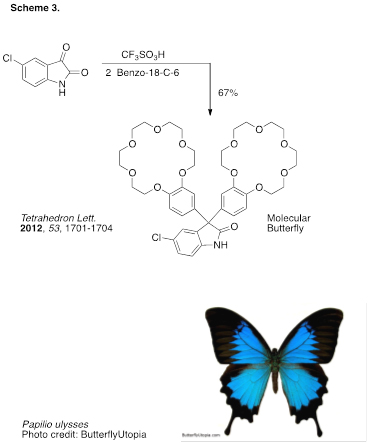
We have also utilized the hydroxyalkylation reaction to provide a variety of bis(benzocrown ethers) – potentially useful metal chelating agents. Using benzocrown ethers, the condensation reactions gave the desired prodoucts in generally good yields (Scheme 3). With dibenzo(crown ethers) and carbonyl substrates, macromolecular products were prepared via A2B2 polymerizations. With their resemblance to butterflies, we have suggested the bis(benzocrown ether) products should be called molecular butterflies. Previous studies in our group have suggested that these types of condensation reactions occur through superelectrophilic processes.
Office
Office: Faraday Hall 356
Phone: (815) 753‐1959
Email: dklumpp@niu.edu
Mailing Address
Prof. Douglas Klumpp
Department of Chemistry and Biochemistry
Northern Illinois University
1425 W. Lincoln Hwy.
DeKalb, IL 60115