Research Interests
Heterocycle Chemistry and Synthesis
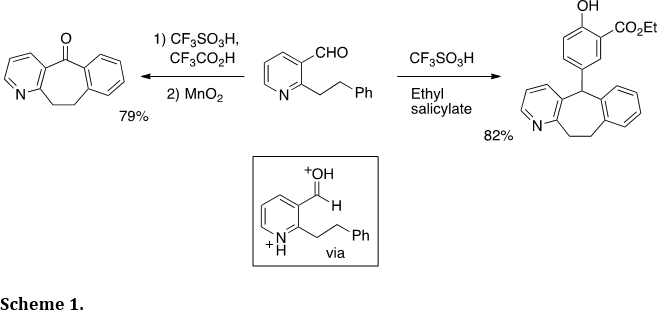
The Klumpp research group has devoted much effort to the development of new synthetic methodologies in the area of heterocycles. With our expertise in acid-catalysis and highly electrophilic intermediates, we have used this chemistry in numerous applications of heterocycle synthesis. Because N-heterocycles often form stable cationic species, the cationic rings may be used to enhance the reactivities of neighboring electrophilic groups. For example (Scheme 1), this was utilized in our synthesis of cycloheptabenzopyridine derivatives (Tetrahedron 2013, 69, 2137-2141). This type of ring system is useful as a pharmaceutical substructure. The diprotonated species is thought to be the initial reactive intermediate.
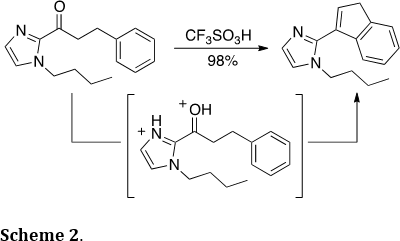
Similarly, heterocycle-substituted indenes could be prepared efficiently through similar chemistry (J. Org. Chem. 2014, 79, 5852-5857). Thus, protonation of the imidazole ring and the carbonyl group leads to the highly reactive carbononium dication, which triggers the condensation reaction (Scheme 2). Similar functionalized indenes have been used as organometallic ligands and pharmaceutical scaffolds.
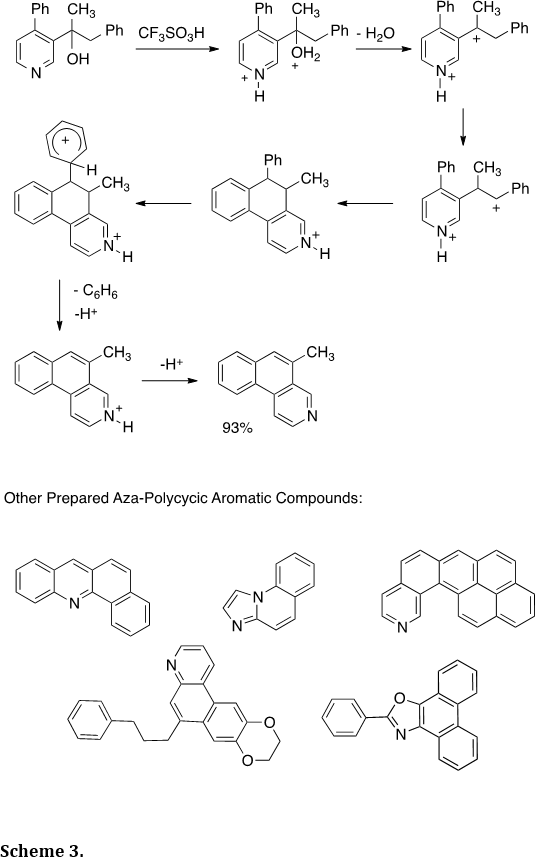
Dicationic intermediates have been shown to be very useful in the synthesis of aza-polycyclic aromatic compounds (Tetrahedron 2012, 68, 3357-3360; Tetrahedron Lett. 2009, 50, 1924-1927; J. Org. Chem. 2008, 73, 3654-3657). As an example, we showed that the pyridyl alcohol provides the benzo[f]isoquinoline in good yield (Scheme 3). During the course of the reaction, the two cationic charge centers are increasingly separated – culminating in the loss of benzene. Charge separation is often a strong driving force in the chemistry of multiply charged species or superelectrophilic intermediates. The chemistry was shown to be wide in scope, as demonstrated by the preparation of a variety of other aza-polycyclic aromatic ring systems (Scheme 3).
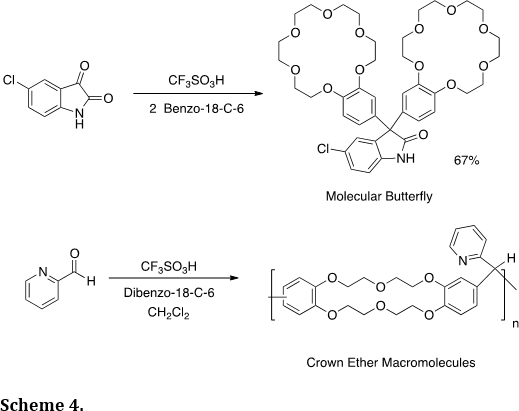
Many of our synthetic methodologies utilize strong or superacidic catalysts. These reagents have enabled use to prepare many novel heterocyclic structures, such as the crown compounds (Tetrahedron Lett. 2012, 53, 1701-1704) – a molecular butterfly and the condensation polymer (Scheme 4).
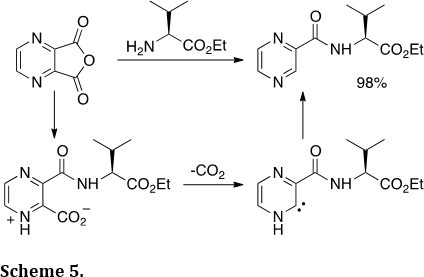
We have examined other reactive intermediates as precursors to heterocycles. For example, we described the synthesis of hetrocyclic amides involving N-heterocyclic carbenes (Org. Lett., 2013, 15, 4806–4809). Reaction of heterocyclic dicarboxylic anhydrides with amines leads to a products from decarboxylation (Scheme 5). The hetrocyclic amide products are valuable scaffolds in drug development.
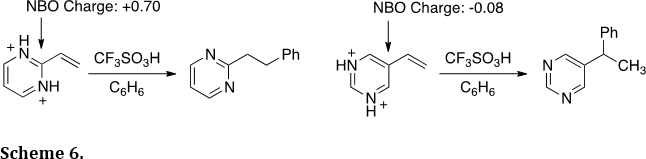
Two recent reports also described regioisomeric effects in the chemistry of vinyl-substituted N-heterocycles (J. Am. Chem. Soc. 2011, 133, 8467-8469; Oranic Lett. 2005, 7, 2505-2508. Two types of addition reactions have been observed in the hydroarylation of vinyl-substituted N-heterocycles. For example, 2-vinylpyrimidine undergoes conjugate addition, while 5-vinylpyrimidine undergoes Markovnikov addition (Scheme 6). Theoretical calculations show the preferred mode of reaction may be predicted by charge distributions in the diprotonated pyrimidine ring. These regioisomeric relationships have been shown for several types of N-heterocycles.
Mechanistic and Physical Organic Studies
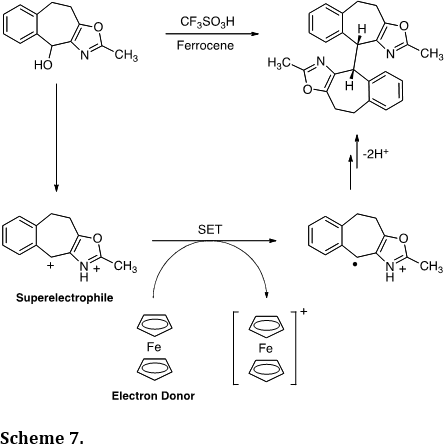
We have carried out a number of mechanistic studies related to cationic reactive intermediates. This includes a report describing the one-electron reduction of dicationic superelectrophiles (J. Am. Chem. Soc. 2008, 130, 14388-14389). Thus, ionization of the oxazole derivative in superacid provides the superelectrophile and single electron transfer (SET) provides the radical cation. Dimerization of the radical centers gives the dimer in >80% yield. Theoretical calculations showed a low energy LUMO on the superelectrophile and this likely facilitates the SET chemistry.
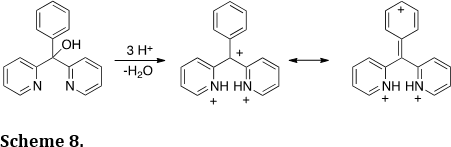
We have used charge-charge repulsive effects to drive chemical processes and predictably move electrons (J. Am. Chem. Soc. 2011, 133, 13169-13175). For example, the tricationic species was generated in superacid (Scheme 8). Charge-charge repulsive effects cause a significant positive charge to form at the para postion of the phenyl ring – a phenomenon verified by NMR spectroscopy, theoretical calculations and nucleophilic trapping studies. Similar charge migration can be promoted by perfluoro-alkyl groups (J. Am. Chem. Soc. 2010, 132, 3266-3267).
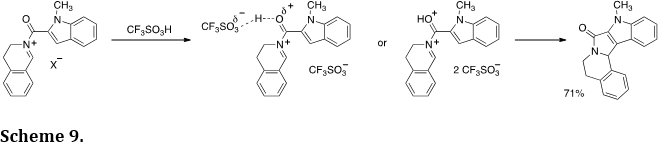
The Nazarov reaction is a useful method of preparing carbocyclic products. Its commonly accepted mechanism involves a 4-p electron electrocyclization reaction step. We hypothesized a similar process should be possible with N-acyliminium ions and the corresponding protonated superelectrophiles (Org. Lett. 2007, 9, 3085-3089; Tetrahedron Lett. 2011, 52, 2195-2198). This led to the development of several examples of the aza-Nazarov reaction (Scheme 9). Theoretical calculations indicate protonation of the carbonyl group may lower the barrier to cyclization by about 12 kcal/mol.
Structural Studies
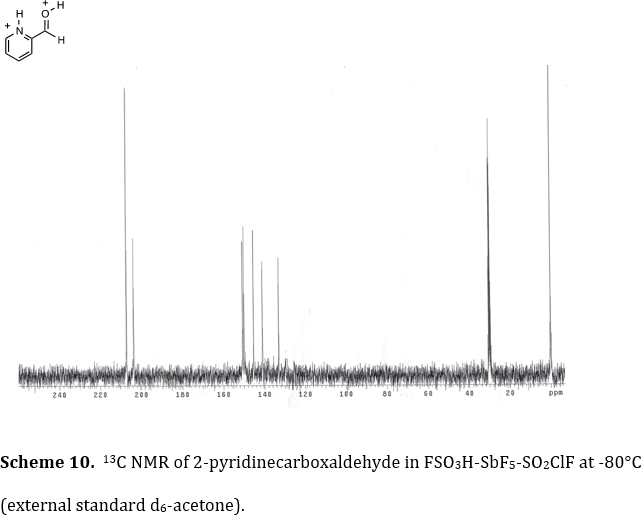
The Klumpp research group utilizes a variety of methods for characterizing reactive intermediates and interesting organic substances. Using the stable ion conditions pioneered by Olah and coworkers, we have been able to directly observe dications, trications and tetracations, by low temperature NMR. This work has characterized several highly reactive species by this spectroscopic method. For example, we observed the diprotonated product from 2-pyridinecarboxaldehyde in FSO3H-SbF5 at -80°C (Tetrahedron 2006, 62, 5915-5921). As seen in Scheme 10, the carboxonium ion carbon 13C signal is visible at d 208.
Many of our studies also take advantage of theoretical calculations. High-level calculations allow us to study reaction energetics, examine the structures of novel intermediates, probe the electronic properties of species and predict the spectra of our reactive ions and molecules. This work has involved collaborations with theoreticians in Brazil, Sweden, Japan and here at Northern Illinois University. Graduate and undergraduate students at NIU have carried out many of these studies.
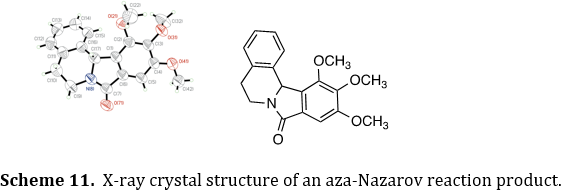
Some of our products are also analyzed by single-crystal X-ray crystallography. This work has been done in collaboration with several crystallographers across the country. The crystallography studies often reveal unusual structural characteristics, such as the helical pitch with the aza-Nazarov product (Scheme 11). Ina few cases, the crystal structures have provided mechanistic insights for our chemical reactions.
Office
Office: Faraday Hall 356
Phone: (815) 753‐1959
Email: dklumpp@niu.edu
Mailing Address
Prof. Douglas Klumpp
Department of Chemistry and Biochemistry
Northern Illinois University
1425 W. Lincoln Hwy.
DeKalb, IL 60115