Heterocyclic Research
The Klumpp research group has been very active in the development of synthetic methodologies leading to functionalized heterocycles. Heterocyclic compounds are an exceptionally important class of organic substances. At least 60% of commercial pharmaceuticals contain a heterocyclic structure.
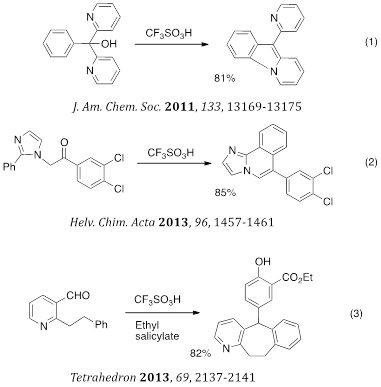
Recent work in the area includes the synthesis of novel ring systems. For example, we utilized the chemistry of superelectrophilic intermediates to prepare a series of pyrido[1,2-a]indoles, Imidazo[1,2-a]pyridines and cycloheptabenzopyridines (eqs 1–3).
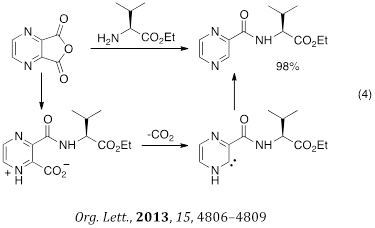
We have also reported the syntheses of heterocyclic carboxamides through a decarboxylation involving N-heterocyclic carbene interemdiates (eq 4). This is a rare example of an NHC being a primary reactive intermediate – as opposed to a catalytic species - in a synthetic conversion.
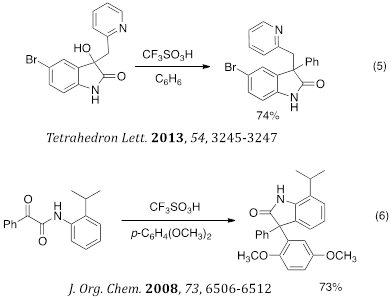
2-Oxyindoles are a class of heterocycles known for their biological activities and we have developed several acid-catalyzed routes to these products (eq 5–6). For example, the 2-pyridyl-substituted system generates a highly reactive electrophile and Friedel-Crafts-type reactions lead to the highly functionalized 2-oxyindoles. We also used a-ketoamides to generate 2-oxyindoles by cyclizations.
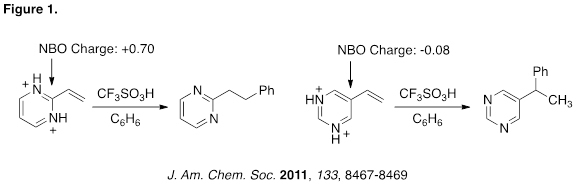
Conjugate addition to vinyl-substituted N-heterocycles has been known since 1947 and it has been used in the synthesis of several natural products and pharmaceutical agents. Recently, I published the first comprehensive review on this topic (SynLett 2012, 23, 1590–1604). In our research lab, we discovered an interesting trend related to conjugate addition to vinyl-substituted N-heterocycles. These studies showed a correlation between the tendency to undergo conjugate addition and the charge distributions in the heterocyclic ring system (Figure 1). The relationship was shown to operate in pyrimidines, quinoxalines and quinazolines, suggesting it to be a general to a wide scope of N-heterocycles.
Office
Office: Faraday Hall 356
Phone: (815) 753‐1959
Email: dklumpp@niu.edu
Mailing Address
Prof. Douglas Klumpp
Department of Chemistry and Biochemistry
Northern Illinois University
1425 W. Lincoln Hwy.
DeKalb, IL 60115