Friedel-Crafts Chemistry Research
The Friedel-Crafts reactions were first described in 1877 and they remain important synthetic conversions leading to functionalized aromatic compounds. Despite being investigated for more than 130 years, Friedel-Crafts-type reactions continue to be actively investigated by academic and industrial researchers. Our own work in this area has involved the development of new synthetic methods and the preparation of useful products.
For example, we recently described the very first use of urea derivatives in Friedel-Crafts acylations. The urea carbonyl group is an exceptionally unreactive electrophile due its resonance interactions with the neighboring nitrogen groups. We have found that this resonance interaction may be “shut down” by suitable groups, such as protonated nitro-substituted aryl groups. Thus, protonation of the nitro group leads to an activated urea and eventually a Friedel-Crafts reaction (eq 1). We demonstrated similar conversions with thioureas. Both intra- and intermolecular reactions were accomplished.
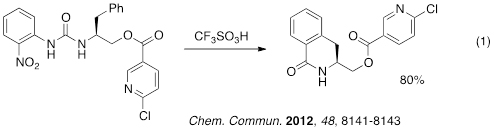
Similar chemistry was described using activated amides – another carbonyl derivative with low reactivity. The product aryl ketones could be prepared by both inter- and intramolecular reactions (eq 2-3) and a reaction cascade provides access to the functionalized tetraline (eq 4). This particular compound is a synthetic intermediate for the anti-depressant drug, sertraline. Both the nitroaniline and the triflic acid may be quantitatively recycled in these synthetic procedures.
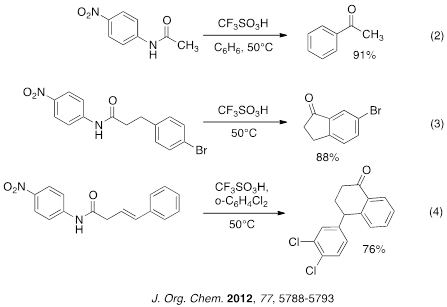
We have also recently described a convenient method for preparing fluorinated ketones through the use of the Houben-Hoesch reaction (eq 5). Fluorinated nitriles generate exceedingly reactive electrophiles using triflic acid catalyst. Following the Friedel-Crafts chemistry, hydrolysis gives the fluorinated ketone products in good to excellent yields.
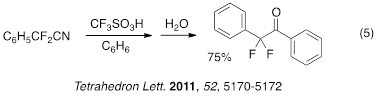
In a series of manuscripts, we have reported novel methods for preparing aza-polycyclic aromatic compounds. A recent manuscript described Friedel-Crafts ring closing followed by superacid-promoted ring opening to give functionalized aza-polycyclic aromatic compounds (eq 6).
Office
Office: Faraday Hall 356
Phone: (815) 753‐1959
Email: dklumpp@niu.edu
Mailing Address
Prof. Douglas Klumpp
Department of Chemistry and Biochemistry
Northern Illinois University
1425 W. Lincoln Hwy.
DeKalb, IL 60115